Module 4: Healthcare practices for common contagious infectious diseases
Ringworm Diagnosis
Since ringworm is a contagious and zoonotic disease, rapid confirmation of true infection is needed for proper treatment that reduces risk for transmission to other susceptible animals and people. No one diagnostic test is considered the gold standard. The best diagnostic approach is to use a combination of the following tools:
- Wood’s lamp exam
- Microscopic exam of plucked hairs (trichogram)
- Fungal culture
- Dermatophyte PCR panel
Wood’s Lamp Exam
-
Wood’s lamp without a magnification lens
-
Wood’s lamp with built-in magnification lens
The Wood’s lamp is a point-of-care (POC) diagnostic tool. A Wood’s lamp exam is considered a core diagnostic test for cats with skin disease. Nearly 100% of M. canis-infected cats have infected hairs that fluoresce a bright apple-green under the Wood’s lamp UV light. A plug-in Wood’s lamp with a wavelength of 320 to 400 nm and built-in magnification should be used.
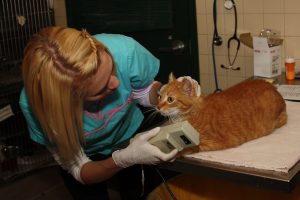
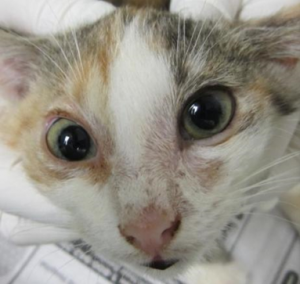
In addition to obvious skin lesions, the Wood’s lamp is used to carefully examine hairs on the face, ears, and feet as these “hot spot” areas may have subtle lesions. Fluorescence in infected hair bulbs and proximal shafts develops within the first week of infection and involves the entire hair shaft within 12 to 14 days. The apple-green fluorescence of M. canis hairs is strikingly distinct from yellow to orange to blue fluorescence of lint, topical medications, doxycycline on the fur, and lime sulfur residue.
-
Wood’s lamp exam shows distinct fluorescent hairs on a cat in the early phase of M. canis infection
-
Wood’s lamp exam shows a large patch of fluorescent hairs in a cat with advanced M. canis infection
-
Cat that is suspicious for ringworm
-
Wood’s lamp exam of the same cat showed fluorescent hairs
In addition to initial diagnosis, the Wood’s lamp exam can be used to monitor response to treatment. Early in the course of an infection, the entire hair will glow, including the hair bulb. As the infection resolves, progressively fewer fluorescent hairs are visible and fluorescence is confined to the distal shafts and eventually only the tips as hairs grow out.
Trichograms
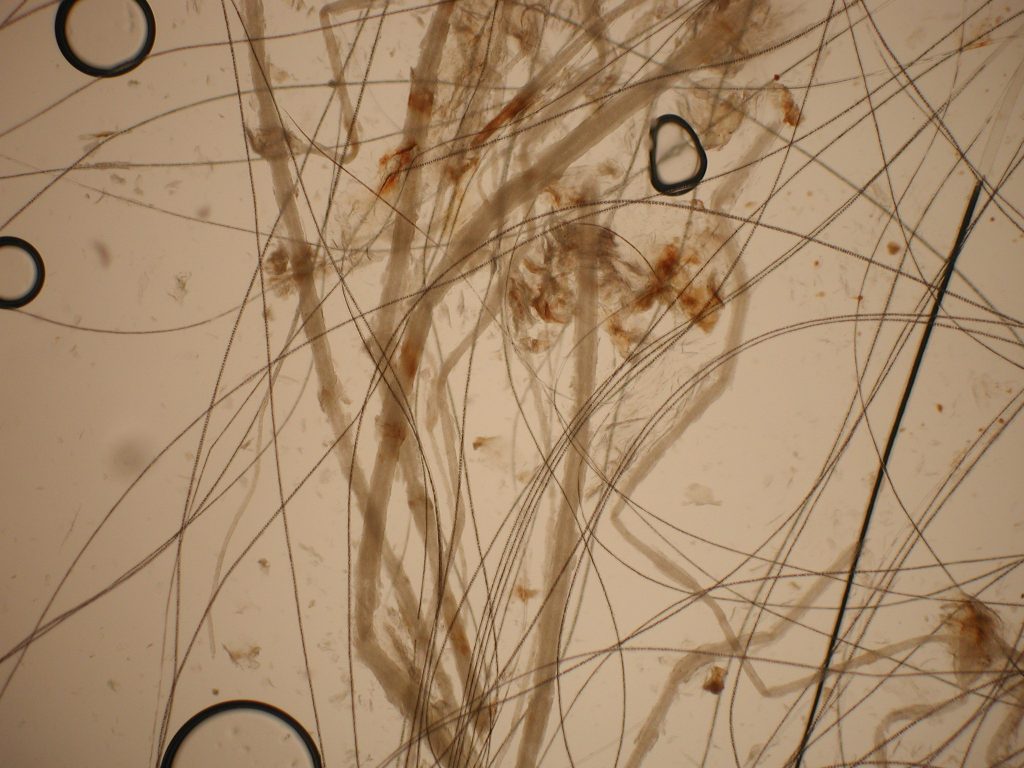
Trichograms, or direct microscopic exam of hairs is another POC diagnostic tool. Plucked hairs from around the lesions are added to a drop of mineral oil on a glass slide. A cover slip is applied for viewing under a microscope using the 10X objective. Infected hairs are much wider than normal hairs due to spores coating the shafts. M canis produces “cuffs” of spores on the outside of infected hairs indicating ectothrix invasion.
In studies comparing Wood’s lamp exams and trichograms with M.canis fungal culture results, the Wood’s lamp exam had a positive predictive value of 90% and a negative predictive value of 94%. The trichogram positive and negative predictive values were 93%. The positive predictive value is the likelihood that a positive result is truly positive, and the negative predictive value is the likelihood that a negative result is truly negative. These studies showed that there is a high correlation between a positive Wood’s lamp examination and trichogram with a positive fungal culture.
Confirming evidence of infection through direct examination with a Wood’s lamp and a trichogram allows ringworm treatment to begin on the day of exam, long before results of a fungal culture are available. Immediate treatment decreases the number of cats exposed and the length of stay for infected cats.
Fungal Culture
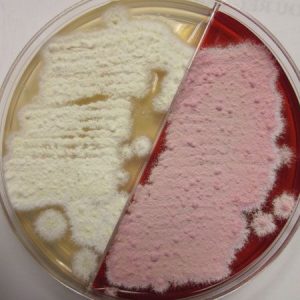
Fungal cultures are performed with dermatophyte test media (DTM) and samples acquired by brushing lesions with new soft-bristled toothbrushes. Multiple lesions should be brushed to collect as many hairs as possible. The toothbrush samples are cultured on POC DTM culture plates or submitted to a reference lab for culturing. POC DTM plates are inoculated by lightly stabbing the toothbrush bristles with hairs onto the surface in 4–5 areas. Culture plates are recommended over DTM vials, as the vial openings are too narrow to pass the toothbrush for inoculation or to sample fungal colonies for microscopic analysis. White fluffy colony growth that turns the DTM media red after a few days is suggestive of a dermatophyte. Studies involving thousands of fungal cultures showed that cultures from truly infected cats are positive by 7 to 10 days, with a median time of 5 to 7 days.
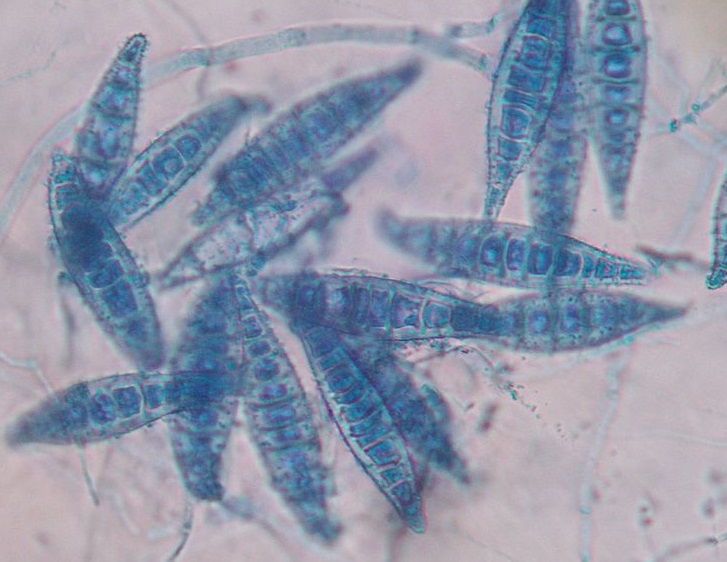
Suspect dermatophyte colonies on DTM plates must be examined microscopically to confirm dermatophyte identification and determine the species. The Scotch tape prep technique is used for microscopic identification where the sticky surface of the tape is touched to the colony and then placed on a drop of blue stain on a slide. M. canis macroconidia are large, spindle-shaped, and thick-walled with six or more internal cells. When microscopic examination is not used for the POC cultures, there is a significant risk for incorrect diagnosis, usually a false positive.
Dermatophyte PCR Panel
Toothbrush samples can also be submitted to Idexx for the Ringworm (Dermatophyte) PCR Panel (test code 3565). The advantages of this very sensitive PCR test are the rapid turnaround time of 2-3 days for diagnosis and identification of the dermatophyte species. A negative PCR result is very reliable and accurate for indicating lack of infection, so it’s especially useful for ruling out infection in cats with suspect skin lesions. Unfortunately, PCR also detects dead spores in samples from treated cats, so it is not useful for monitoring response to treatment or determining if a cat is cured.
Fomite or Dust Mop Cats
Some cats test false positive with Wood’s lamp, trichogram, fungal culture, or PCR testing due to M canis spore carriage on their fur from contact with an infected animal or exposure to a contaminated environment. These “fomite” or “dust mop” cats are not truly infected and usually do not have any skin lesions. They should be treated once with a lime sulfur dip to kill the fungus and moved on to placement. They do not need to be isolated or treated.
Test Your Knowledge